A Carbon/Graphite Engineer Explains
As bushings and mechanical seals need to be replaced or are required to withstand higher pressures and temperatures, more engineers are choosing to use carbon materials for their mechanical components.
Specifically, engineers gravitate to carbon graphite and graphite materials. While carbon graphite and graphite can both help improve application performance, their compositional build up make them unique from one another and each offer unique properties to different applications.
To determine how the material benefits an application, it can be helpful to start with the question ‘What is carbon graphite?’ A common misconception is that carbon graphite and graphite are the same thing. The simple answer is ‘No, carbon graphite and graphite are not the same.’ Carbon graphite and graphite differ in their chemical structures; therefore, they also differ in their properties.
What Is Carbon Graphite?
In chemistry, we use a solid’s arrangement of atoms to learn about and explain the properties of the material. Let’s take a look at carbon’s atomic structure and its allotropes.
When speaking about carbon, there are a number of allotropes, or arrangements of atoms, that can exist. Two of the most common are diamond and graphite.
Diamond is formed when a carbon atoms bonds covalently with three other carbon atoms. This creates a tetrahedral shape where one carbon atom is bonded to three other carbon atoms to form a pyramid as seen in Figure 1.
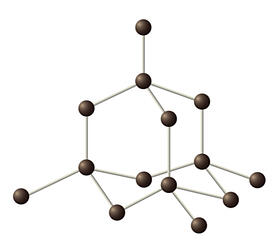
Figure 1. Diamond atomic structure
Graphite, on the other hand, is formed when one carbon atom bonds covalently with three other carbon atoms, leaving one valence electron free. This forms a hexagonal crystalline structure where the bonded carbons form a plane which is referred to as graphene. Each graphene layer is connected by Van der Waals forces which are significantly weaker than the carbon to carbon covalent bonds. Graphite’s chemical structure can be seen in Figure 2.
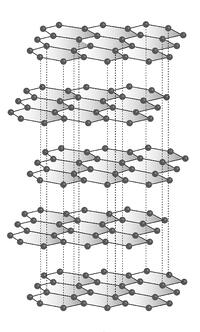
Figure 2.Graphite hexagonal crystalline atomic structure
Unlike diamond and graphite, amorphous solids do not have a crystalline structure or predetermined atomic shape. This difference is depicted in Figure 3.
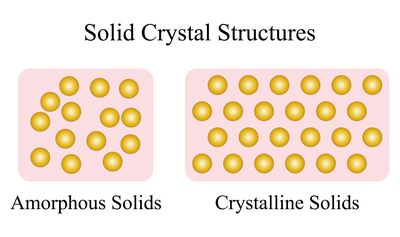
Figure 3. Representation of amorphous solids compared to crystalline solids
Solids have precise crystalline structures, as in the right image in Figure 3, whereas amorphous solids do not, as the left image in Figure 3. Here, atoms are sporadically arranged with no consistent spacing or placement. Thus, bond strengths and distances vary throughout the material.
As a result, amorphous carbon can be depicted as a messy and intertwined layout as seen in Figure 4. This intertwined layout can be rearranged into another previously discussed carbon allotrope, graphite, when heated to temperatures up to 3000⁰C.
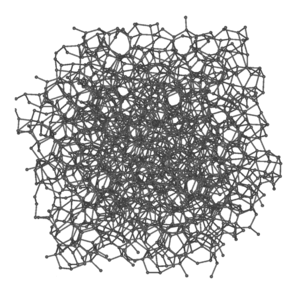
Figure 4. Amorphous carbon representation
The combination of amorphous carbon and graphite is what makes up “carbon graphite.” This is obtained after baking a mixture of graphite with a carbon binder. Because amorphous carbon can be rearranged into graphite, the carbon graphite mixture can be further processed to obtain graphite through graphitization.
What Is Graphitization?
Graphitization is the process of heating amorphous carbon for a prolonged period of time, rearranging the atomic structure to achieve an ordered crystalline structure that is typical of solids. During graphitization, carbon atoms are rearranged to fill atom vacancies and improve atom layout.
The rearranging of atoms in amorphous carbon is enhanced in the presence of oxidizing gases. This allows for the breaking of bonds in more disordered regions of the amorphous carbon. This includes locations where carbon to carbon bonds can be interlinked between layered planes or other intertwined areas of the amorphous structure.
The Graphitization Process
The graphitization process requires temperatures up to 3000⁰C. As temperatures increase, the following takes place:
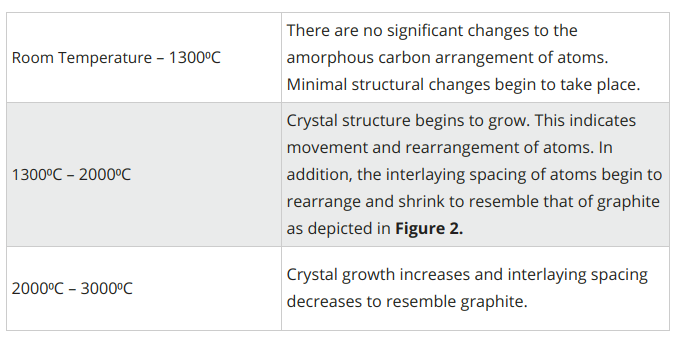
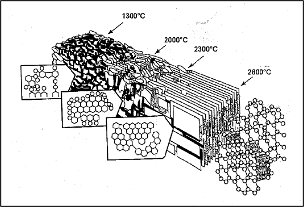
Figure 5. Evolution of graphitization process1
Results Of Graphitization
As the temperature increases during graphitization and the crystal structure gets closer to graphite, the mechanical properties begin to change as well and approach values closer to that of graphite.
As a result of graphitization, the atoms rearrange to the crystal structure of graphite. The layered structure of graphite is responsible for increasing lubricating properties, oxidation resistance, and thermal properties as seen in Table 1.
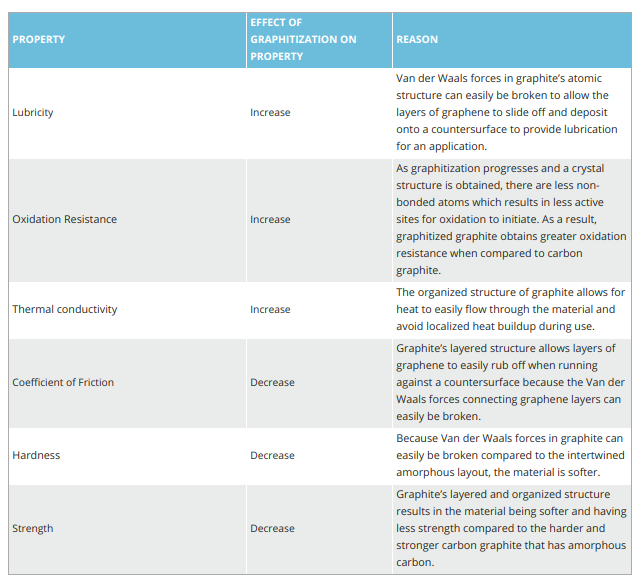
Material grades can be impregnated as carbon graphite or graphite. One base may be more beneficial than the other depending on the application parameters.
When Is Graphite A Recommended Material?
Graphite is typically better in the following applications:
- Dry Running Applications Graphite is more lubricious than carbon graphite and the coefficient of friction is lower, so less heat will be generated during use, making it perfect for dry running applications.
- Oxidizing Applications Graphite is more resistant to oxidation than carbon graphite. When paired with an oxidation inhibitor impregnation, the temperature resistance is higher than a carbon graphite.
- Chemical Applications Graphite acts as an inert material which is required to prevent corrosion. The chemical resistance can increase depending on the impregnation.
While these are some applications where graphite performs well in, material grades are not limited to specific applications.
Carbon graphite and graphite both have valuable properties when using them for mechanical applications. However, based on the application and environmental parameters, it can be more beneficial to use one over the other.
For more information or to purchase wear rings, contact a carbon graphite manufacturer today.
References
- Delhaes, P., 2012. Carbon Science and Technology – From Energy To Materials. Hoboken, NJ: John Wiley & Sons, Inc., pp.35-38.
- Mantell, C., 1968. Carbon and Graphite Handbook. John Wiley & Sons, Inc., pp.6-14.
- Hoge, Keith. “From Powders To Parts: A Look Into The Carbon Graphite Production Process (Part I)”. Blog.Metcar.Com, 2020, https://blog.metcar.com/carbon-graphite-production-process-part-1. Accessed 3 Aug 2020.Blog
- Hoge, Keith. “From Powders To Parts: The Carbon Graphite Production Process (Part II)”. Blog.Metcar.Com, 2020, https://blog.metcar.com/carbon-graphite-production-process-part-2. Accessed 3 Aug 2020.

![Durlon Chemap® filters solve filtration tasks in a simpleand economic way [Case Study]](https://test.empoweringpumps.com/wp-content/uploads/2022/12/Durlon-Chemap®-filters-solve-filtration-tasks-in-a-simpleand-economic-way-Case-Study-7-380x199.png)


Comments